Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Retaliating Properties of Naringin: A Mini-Review
*Corresponding author:Payal Mahobiya, Endocrinology Laboratory, Zoology Department, Dr Harisingh Gour Vishwavidyalaya, Sagar, MP, India.
Received:December 04, 2022; Published:December 14, 2022
DOI: 10.34297/AJBSR.2022.17.002381
Abstract
Plants contain a flavonoid called naringin, which has a variety of medicinal uses. Tomatoes, grapefruits, and other citrus fruits contain the flavanone glycoside known as 4′,5,7-trihydroxyflavanone-7-rhamnoglucoside. There are seven enzyme-catalyzed stages in the phenylpropanoid route, which runs from phenylalanine to prunin. Naringin possesses anti-inflammatory, anti-tumor, and antioxidant effects. Naringin has been shown to have impacts on tumours, osteoclastogenesis, and asthma in both in vivo and in vitro studies using a range of test animals and cell lines. In vitro and in vivo studies have proven the effectiveness of naringin in a number of preclinical models of atherosclerosis, cardiovascular disease, neurodegenerative disease, osteoporosis, and rheumatological illnesses. Naringin may be a useful natural medication for treating human metabolic diseases, according to research on animals. Since there are few studies on naringin in humans, this review concentrates on the substance’s various known activities in in-vitro and in vivo preclinical models as well as its potential therapeutic uses based on what is currently understood, such as its bone regeneration, anti-inflammatory, and antioxidant properties. But additional analysis of naringin’s impact on people is required.
Keywords: Naringin; Antioxidant; Anti-inflammatory; Bone regeneration; Medical applications
Introduction
Flavonoids are naturally occurring phenolic compounds having a wide range of bioactivities. Most the fruits, vegetables, and herbs contain flavonoids. The flavonoid structure is composed of three rings having 15 carbon atoms, two of which are heterocyclic rings linked together by a three-carbon chain. Flavanones, flavones, isoflavones, flavanols, anthocyanidins, and flavanols are all flavonoids found in plants. In the body, these flavonoids act as free radical scavengers, preventing oxidative stress [1]. Albert Szent- Gyorgi, a Nobel Prize-winning biochemist, discovered and named them “Vitamin P.” Bioflavonoids are polyphenolic phytochemicals. Polyphenolic acids, flavonoids, stilbenes, and lignans are examples of major polyphenols. The most abundant polyphenols in our diets are flavonoids [2]. Citrus fruits may help with diabetes and obesity treatment. Flavonoids are potent antioxidants that encourage the strengthening of capillary walls, the prevention of bruising and bleeding, the protection against free radicals, and the improvement of circulation. Some flavonoids are powerful anti-inflammatory agents that aid in tissue repair [3]. In human umbilical vein endothelial cells, citrus fruit extract demonstrated significant antioxidant activity [4].
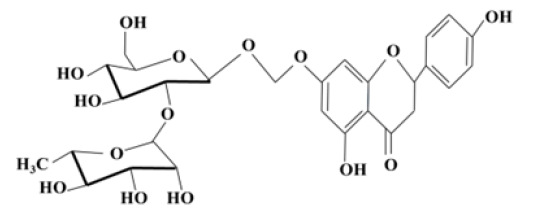
Naringin (Figure 1), one of the main active components of Chinese herbal medicines is a flavanone glycoside formed from the flavanone naringenin and the disaccharide neohesperidose. such as Drynaria fortunei (Kunze) J. Sm. (DF), Citrus aurantium L. (CA) and Citrus medica L. (CM) [5,6]. The naringin content of different fruits varies as follows: CA>Immature CA, Immature Ponciri Fructus>Citri Unshiu peel>Immature Citri Unshiu peel [7]. Naringin’s molecular formula is C27H32O14, and its molecular weight is 580.4 g/mol. It is also found in citrus fruits and gives citrus juices a bitter taste [8].
Naringin and naringenin are both powerful antioxidants [9,10] whereas, because of the sugar moiety in the latter, naringenin is more potent than naringin. Naringin is water soluble. The gut microflora degrades naringin to its aglycon naringenin, which is then absorbed [11]. Naringin is gaining popularity as a result of its synergistic activity with a variety of supplements and potential drugs. It enhances nutrient absorption so after supplementation. There are no known flavonoid deficiencies. Kanokorn et al. [12], recently described a simple and high-yield method for extracting and purifying naringin from agricultural wastes including citrus fruit peels. Naringin formulations with controlled release may reduce overall dose required, and these formulations should have high encapsulation efficiencies and stable drug release behaviors [13]. Naringin formulations with controlled release have also been shown to significantly affect bone regeneration and possibly promote bone healing [14].
Flavonoids are a type of plant secondary metabolite that is high in bioactive compounds. According to a comprehensive literature review, naringin has antioxidant, anti-inflammatory, anti-apoptotic, anti-ulcer, anti-osteoporotic, and anti-carcinogenic properties [15]. However, until recently, there had been few reports describing naringin processing. The current review focuses on recent studies that describe the in vivo and in vitro effects of naringin, emphasizing the compound’s potential value and breadth of pharmacological activities.
Naringin in Grapefruit Seed
Grapefruit seed extract has antibacterial, antiviral, antifungal, and antifungal properties. It is used as a first-aid treatment, a facial cleanser, a vaginal douche, a skin irritation remedy, a gargle for sore throats, an ear or nasal rinse to treat infections, a dental rinse, and a breath freshener. To treat lung infections, its vapor can be inhaled. It is used in agriculture to kill fungus, bacteria, and parasites in animal feeds, to combat mould growth, to disinfect water, and to preserve food [16].
Bio Application of Naringin
Naringin is a drug that is used to treat diabetes, herpes, heart failure, alcoholism, and chronic venous insufficiency. Naringin is frequently used in the nutrition industry to boost the absorption of supplements like caffeine [17]. It enhances ethanol metabolism and mitigates the negative effects of ethanol consumption; it acts as an antioxidant and free radical scavenger; and it significantly inhibits LDL oxidation. Reduces cytotoxicity, acts as an anti-apoptotic agent, and has antifungal properties. Naringin inhibits the inhibition of endogenous, autophagic-lysosomal protein degradation caused by okadaic acid, as well as receptor-mediated glycoprotein uptake and degradation. Naringin can be used to prevent pathological hyperphosphorylation, chemotherapeutic drug side effects, and environmental toxins. Naringin (25 mg) increases the bioavailability of nutrients and drugs [18]. The effect of naringin on the pharmacokinetics of quinine was studied in female Wister rats weighing 190-200g via oral administration. There was no change in pharmacokinetic parameters for IV administration, but a significant increase for oral administration. Pretreatment with naringin increased Cmax by 20.8 percent, AUC by 15.2 percent, and bioavailability by 17 percent to 42 percent. The findings confirmed that pre-treatment with naringin improves drug pharmacokinetics [19].
Extraction method
Three steps are required to isolate naringin from fruits: extraction, separation, and purification. The naringin content of fruit is affected by several factors, including harvest time, fruit part used, and drying time if the peel is the source of naringin. A convection oven can be used to dry the peel faster than the sun, reduce aerial exposure time, and prevent microbial activity, which could result in naringin destruction and metabolite contamination [20]. In previous studies, CM powder (0.5 g) was extracted with 50% methanol (25 mL) for 30 min using ultrasonication [21] and CA powder (100 g) was extracted by refluxing with methanol (1L) for 2 hours, which can help to retard or eliminate microbial infection. The CA extract yielded 25.8 percent naringin when redissolved in methanol, yielding a crude drug solution with a final concentration of 0.1 g/mL (5). Methanol extraction was followed by crystallization with water at 25°C containing 14-15 percent (v/v) dichloromethane, yielding a fivefold increase over conventional hot water extraction. From 1kg of dry pomelo peel, this method yielded 20 g of naringin (>98 percent purity). The structure of naringin was validated using ultraviolet-visible spectroscopy (UV-VIS), Fourier transform-infrared spectroscopy (FTIR), 1H NMR spectroscopy, mass spectrometry, and elemental analysis after concentration [22].
Naringin biosynthesis via phenylpropanoid pathway
The phenylpropanoid pathway begins with essential amino acid, associate effect of shikimate pathway. The phenylpropanoid pathway offers rise to a diversity of finish merchandise starting from flavonoids, tannins and lignins [23]. The portrayal of phenylpropanoid path is conferred henceforth.
The foremost seven enzyme catalyzed phases of phenylpropanoid biosynthesis pathway primes to naringin synthesis (Figure 2). In the initial step, conversion of phenylalanine into cinnamic acid by enzyme phenylalanine ammonia-lyase (PAL) occurs. Deamination of phenylalanine fetches out to form cinnamic acid and ammonia. In next step, cinnamate 4-hydroxylase (C4H) initiate conversion of cinnamic acid into p-coumarate. p-coumarate is then processed into p-coumaroyl CoA via enzyme 4-coumarate CoA-ligase (4CL) [24]. The trail up to p-coumaroyl CoA synthesis is over-all phenylpropanoid pathway. Afterward, the pathway branches out into flavonoids, stilbenes, proanthocyanin’s, Flavonols and anthocyanins. The enzymes chalcone synthase (CHS) and chalcone isomerase (CHI) catalyze the separation of phenylpropanoids into flavonoid biosynthesis. Additionally, uridine diphosphoglucoseflavanone 7-O-glucotransferase (UF7GT) arbitrated catalysis engenders a group of miscellaneous metabolites [25].
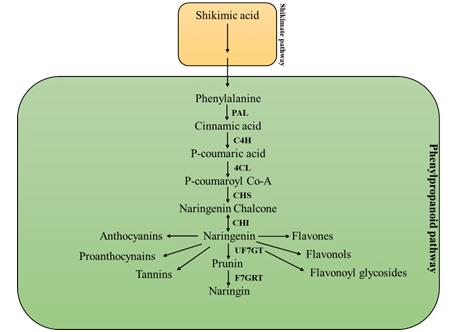
Figure 2:Brief outline of phenylpropanoid biosynthesis pathway. The shikimate pathway primes to the synthesis of phenylalanine, act as initial molecule of the phenylpropanoid biosynthesis pathway. Phenylalanine is processed into naringin via seven enzyme-catalysed steps. The enzymes truncated as PAL, C4H, 4CL, CHS, CHI, UF7GT and F7GRT stands for phenylalanine ammonia lyase, cinnamate-4 hydroxylase, 4-coumaroyl: CoA-ligase, chalcone synthase, chalcone isomerase, uridine diphosphoglucose-flavanone 7-O-glucosyltransferase and flavanone 7-O-glucoside 2-O-beta-L-rhamnosyltransferase, respectively
Effects on bone regeneration and bone marrow protective
In both intact and gonadectomized animals, naringin has a significant effect on bone repair. It has been demonstrated to increase BMD and bone strength while inhibiting urinary calcium excretion. Naringin inhibited the OVX-induced increase in urinary calcium excretion as well as bone mass and strength losses in ovariectomized (OVX) mice, and it improved bone quality in the distal femur, proximal tibia, and lumbar spine. By modulating OPG and RANKL expression, naringin and the DF flavonoid fraction may mimic oestrogen and suppress osteoclastgenesis in osteoblastic cells (Figure 3) [26]. Naringin has been shown to significantly increase plasma insulin-like growth factor 1 levels, femoral BMD and strength, fifth lumbar spine BMD, and femur and lumbar spine bone calcium concentrations in young retinoic acid-induced osteoporosis rats [27]. Naringin (200 mg/kg by oral gavages) has been shown in OVX rats to increase BMD, bone volume, trabecular thickness, and maximum load (28). Naringin has statin-like effects on lipid reduction, BMP stimulation, and the bone-fat mass relationship in gonad-intact aged male rats. However, naringin supplementation only affects metaphyseal density; it has no effect on the diaphyseal region [29].
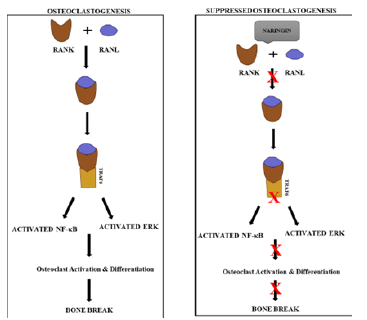
Figure 3:Naringin’s regulatory effect on bone metabolism, Osteoclast activation causes bone regeneration, which leads to bone fracture. Binding of RANK to RANL activates NF-B and ERK, promoting osteoclast formation. Naringin inhibits RANK-RANL binding, thereby inhibiting downstream activations and counteracting osteoclast formation.
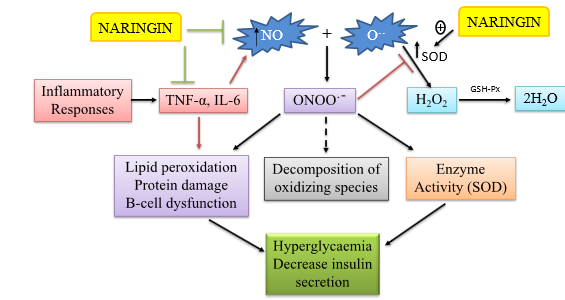
Figure 4:Nitric oxide, oxidants, the antioxidant SOD, and the action of naringin are represented schematically. When inflammatory cytokines like interleukin (IL) and TNF are released, inducible nitric oxide synthase activity is increased, which can lead to an excess of NO being produced. However, the antioxidant enzyme superoxide dismutase (SOD) turns oxygen into hydrogen peroxide (H2O2), which is then further neutralized by other antioxidant enzymes. A variety of conditions, including lipid peroxidation, oxidative stress, protein damage, hyperglycemia, and insulin resistance, may result from this extra nitric oxide’s interaction with the free oxygen radical superoxide anion (O−2∙).
Naringin has been shown in other research models to be effective. Treatment with naringin by oral gavage (300 mg/kg daily) for 30 days or by local injection in mice with PMMA-induced osteoclastogenesis has been shown to ameliorate the PMMA-induced inflammatory tissue response and subsequent bone resorption, as well as to significantly alleviate periprosthetic bone resorption [30]. Treatment with naringin (400 mg/kg) by gastric perfusion for 10, 20, or 30 days improved alveolar bone regeneration in rats with LPS-induced alveolar bone resorption, but the effect was less pronounced in vivo than in vitro. Naringin improved alveolar bone regeneration in ten-week-old male Sprague-Dawley rats, with the greatest effect seen on day 30 [31]. Naringin treatment for 10–30 days can improve bone regeneration, BMD, and bone strength in animal models, but the effects of the dose and administration route, as well as the framework of action and side effects, are unidentified.
Naringin was tested to see if it could shield bone marrow from gamma radiation. In the study, mice were pre-treated with naringin before being exposed to 2Gy of 60 Co gamma radiation. The effects of radiation on micro nucleated polychromatic (MPCE) and normochromic (MNCE) erythrocytes were studied. A 2mg/kg dose of naringin pre-treatment reduced the formation of MPCE, MNCE, and complex chromosome aberrations in mice. The findings show that naringin protects against radiation-induced DNA damage and inhibits cell proliferation [32].
Anti-oxidant effects
Phenolic phytochemicals are considered to benefit health in part by acting as antioxidants and scavenging free radicals. Toxicity can be caused by disruptions in the normal redox state of cells, which produce ROS and free radicals that damage all of the cell’s components [33]. Naringin has been shown to have dosedependent radical scavenging activity against radicals such as 1,1-diphenyl-2-picryl-hydrazyl and tetraethylammonium chloride. In Chinese hamster fibroblast (V79) cells, naringin demonstrated antioxidant activity and reduced the frequency of H2O2-induced DNA damage at concentrations ranging from 5 to 2000μM [34]. Pretreatment with naringin (3 or 24 h) reversed the decrease in glutathione (GSH) and increases in both intracellular free radicals and glucose uptake in IL-6 myoblast cells subjected to oxidative stress. Naringin (100μM) also resulted in a 40% decrease in protein glycation [35]. Naringin (10μM) activated the Nrf2 signaling pathway in the rat glomerular mesangial cell line HBZY-1, increasing the expression and activity of its downstream target, HO-1 [36]. In aged rats, naringin (25, 50, or 100 mg/kg) significantly and dose-dependently prevented all of the biochemical and molecular changes caused by cisplatin [8]. Naringin reduces oxidative stress in pentylenetetrazole-induced seizure rats by binding free radicals and regulating GSH levels. Pretreatment with naringin for several hours has been shown to significantly reduce pentylenetetrazoleinduced increases in malondialdehyde and TNF- levels in the brain, as well as to conserve GSH [37]. Naringin reduced diabetes-induced lipid peroxidation and ROS accumulation in sperm, as well as the minimization in the GSH: oxidized GSH ratio in diabetic rats [38]. Naringin increased the activity of the antioxidant defense system in HFD/STZ-induced diabetic rats with hyperglycemia-induced oxidative damage, conferring protection by reducing the activities of hepatic SOD, GSH reductase, GSH peroxidase, and catalase [39]. Chronic administration of naringin for 6 weeks attenuated oxidative damage in D-galactose-treated mice by decreasing lipid peroxidation and nitrite concentrations and restoring the lowered GSH level as well as SOD, catalase, and GSH-S-transferase activities [40]. Hyperglycemia may be responsible for the increased oxidative stress owing to the increase in level of nitric oxide and reduction of SOD. Naringin did not only ameliorate the hyperglycemia, but also improved the insulin secretion. Naringin constrains inflammatory mediators like TNF-α and IL-6 further regulating nitric oxide production and improving antioxidant levels of SOD [41].
Anti-Inflammatory Effects
Inflammation is a component of the complex cellular predisposition of vascular tissues to harmful stimuli such as pathogens, damaged cells, or irritants [42]. Even though inflammation is a reasonable response to tissue injury, if left uncontrolled, it can lead to chronic autoimmune diseases, and anti-inflammatory compounds may be required to control the inflammatory response. Plants high in flavanones, such as hesperidin, naringin, and neohesperidin, have long been used to treat inflammation [43,44]. Naringin did not inhibit cellular proliferation but did prevent the formation of RANTES (regulated upon activation normal T-cell expressed and secreted) in a human epidermal keratinocyte cell line by inhibiting the nuclear translocation of NF-B. (HaCaT cells). RANTES have been shown to be modulated by tumour necrosis factor alpha (TNF-α)/interferon-β (IFN-β) at 24 and 48 hours. When stimulated by TNF-α/IFN-β for 48 hours, HaCaT cells produce more RANTES than when stimulated for 24 hours [45]. Naringin has been shown in animal models of inflammation to be effective in reducing the expression of signaling factors associated with the inflammatory response, such as interleukin-6 (IL-6), interleukin-8 (IL-8), inducible nitric oxide synthase (iNOS), nuclear factor erythroid 2-related factor 2 (Nrf2), and TNF-α.
Treatment with naringin in 20-month-old male Wistar rats may have prevented an increase in serum Interleukin-6 all through aging-related inflammation [29]. Furthermore, naringin suppressed LPS-induced iNOS expression and NO production in macrophages [46]. Naringin reduced the concentrations of IL-8 and leukotriene B4 in bronchoalveolar lavage fluid (BALF) and decreased myeloperoxidase activity in both the BALF and lung tissue in a guinea pig model of chronic bronchitis. It also increased superoxide dismutase (SOD) activity in lung tissue and increased the level of lipoxin A4 in BALF [47]. Naringin may reduce inflammation in rats with 3-nitropropionic acid (3-NP)-induced Huntington’s disease by modulating Nrf2-driven ARE gene expression and decreasing TNF-α, COX-2, and iNOS expression. The health implications of naringin in 3-NP-induced inflammation are due to Nrf2-mediated gene expression upregulation, which reduces the production of pro-inflammatory mediators [48].
Naringin forms a 1:1 complex with Cu (II), with the Cu (II) ion coordinated via naringin positions 4 and 5. While maintaining cell viability, the naringin-Cu (II) complex demonstrated greater antiinflammatory activity than free naringin [22]. Painopowder is an ancient Chinese medicine that contains the active ingredients naringin, paeoniflorin, neohesperidin, and platycodin-D. The fouringredient combination produced the greatest anti-inflammatory effect in a model of acute inflammation, but naringin was found to contribute the most to the effect of the four ingredients [31].
Other Activites
Effects on genetic damage
Some reagents and medications, such as H2O2 and lomefloxacin, cause DNA damage that, if not repaired, can lead to genetic mutations and/or genomic instability [49]. In V79 cells, naringin exhibits antioxidant properties and protects against H2O2-induced chromosome breakage and loss, as well as DNA damage. Naringin may protect against H2O2-induced oxidative damage by decreasing DNA damage while increasing DNA repair potential [34]. Naringin pretreatment has been shown to reduce lomefloxacin-induced genomic instability in mice. At concentrations ranging from 5 to 50 mg/kg, naringin significantly reduced cell proliferation, chromosomal aberrations, and micronucleus formation in bone marrow while increasing mitotic activity [50]. Naringin has been shown to modulate the production and expression of oxidative mediators as well as DNA damage in male Wistar rats, alleviating the symptoms of inflammatory bowel disease [40]. Naringin treatment for four weeks significantly reduced the rate of DNA strand breaks in diabetic rats. The inhibition of hyperglycemia-induced free radical generation by naringin mediates its anti-genotoxic effect [38]. Naringin has anti-genotoxic properties and reduces DNA damage by modulating the expression of oxidative arbitrators and free radical production.
Effect of Naringin on hypertension
Naringin supplements have a strong antihypertensive effect in high-carbohydrate, fat-fed obese rats and stroke-prone hypertensive rats. Nitric oxide metabolites were found in the urine of animals given naringin [51]. In high-carbohydrate fat-fed obese rats and streptozotocin-induced diabetic rats, it also acts as a vasodilator [52].
DNA repair
Prostate cancer risk increases with age. Naringin has been shown to repair damaged DNA in human prostate cancer cells. Because it removes potentially cancer-causing mutations in cells, DNA repair is one of the body’s primary defence mechanisms. Naringin promotes DNA health by activating two DNA repair enzymes during replication. DNA polymerase beta (DNA poly beta) and 8-oxoguanine-DNA glycosylase 1 (hOGG1) are both enzymes involved in the DNA base excision repair (BER) pathway. Prostate cancer starts slowly and grows undetected. Regularly consuming grapefruit may help to prevent its progression by promoting the repair of damaged DNA in prostate cells [53], naringin was found to be effective in protecting lipids from oxidative damage. Both flavanones reduced DNA damage in the same way [54].
Effects on central nervous system (CNS) diseases
Naringin has been shown to help with a variety of CNS diseases, including Alzheimer’s, Parkinson’s, and epilepsy [55]. Naringin was found to cause significant increases in malondialdehyde and nitrite levels, as well as significant decreases in GSH levels and SOD and catalase enzymatic activities in rats treated intracerebroventricularly with streptozotocin (ICV-STZ rats). Naringin also reduces acetylcholinesterase activity and TNFlevels
in ICV-STZ rats’ brains [56]. In a Parkinson’s disease rat model, intraperitoneal administration of naringin protects the nigrostriatal dopaminergic (DA) projection by increasing glial cell line-derived neurotrophic factor expression and decreasing TNF-α expression in DA neurons and microglia [57]. Naringin treatment significantly reduced seizure in KA-treated mice. Furthermore, it protected hippocampal CA1 neurons in the KAtreated hippocampus, alleviated KA-induced autophagic stress, reduced microtubule-associated protein light chain 3 (LC3) expression and dampened an improvement in TNF-α levels [58]. Naringin significantly improved learning and memory in mice fed an HFD for 20 weeks, as evidenced by a 52.5 percent improvement in the recognition index, a 1.05-fold increase in the crossing-target number, and amelioration of mitochondrial dysfunction. In a mouse model of Alzheimer’s disease, naringin (100 mg/kg/day) increased Thr286 phosphorylation by 47% when compared to untreated APPswe/PS1dE9 mice, indicating that naringin improves CaMKII autophosphorylation and function [59].Conclusion
The real facts and various biological applications behind the naringin were studied. The review concludes with the impression that the flavonoid has various applications as dietary supplement and has various therapeutic activities. Since it exhibits the bio enhancer property it has to be taken with little caution only on treatment with any drug if not it can be added in our daily diet which keep and make the body healthier.
Thus, the data suggests that naringin has therapeutic potential in a variety of human disorders. Nonetheless, the use of naringin in clinical therapy is fraught with numerous flaws as of today. To begin with, there is very little data on the use of naringin in humans, and as a result, the precise effect of naringin in these human disorders, if any, can only be predicted. As a result, additional clinical studies are required to establish a conclusive role for naringin in human therapeutics. Second, naringin is a natural dietary component. As a result, regular food consumption would undoubtedly introduce naringin into the human body, but it is unclear whether this administration is sufficient to meet therapeutic levels in humans, or if additional external supplementation is required. Naringin is a favorable therapeutic approach as antioxidant and anti-inflammatory natural component, demanding additional investigation in metabolic disorders, as well as in other remedial conditions where such pathophysiological variations are apparent. Moreover, the duration of naringin administration is also unknown, as naringin administration for a short period of time is unlikely to result in therapeutic improvement. Naringin’s effect can only be obtained by ingesting it continuously. Third, when used in conjunction with other allopathic medications, the significant potential for drug interactions with naringin should be considered. Nonetheless, naringin appears to be the light at the end of the tunnel as a supportive remedy for allopathic treatment, given its broad range of purported efficacy and lower incidence of adverse reactions.
Acknowledgment
This research work was financially assisted by UGC NON-NET FELLOWSHIP for carrying out this research work successfully. The authors wish to thank the Department of Zoology, School of Biological Sciences, Dr. Harisingh Gour Vishwavidyalaya, Sagar, M.P., India, for providing infrastructure facilities.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
References
- Peterson J, Dwyer J (1998) Flavonoids: dietary occurrence and biochemical activity. Nutrition research 18(12): 1995-2018.
- Nijveldt RJ, Van Nood E, Van Hoorn DE, Boelens PG, Van Norren K, et al. (2001) Flavonoids: a review of probable mechanisms of action and potential applications. The American journal of clinical nutrition 74(4): 418-425.
- Fuhr U, Kummert AL (1995) The fate of naringin in humans: A key to grapefruit juice‐drug interactions?. Clinical Pharmacology & Therapeutics 58(4): 365-373.
- Trombetta D, Cimino F, Cristani M, Mandalari G, Saija A, et al. (2010) In vitro protective effects of two extracts from bergamot peels on human endothelial cells exposed to tumor necrosis factor-α (TNF-α). Journal of agricultural and food chemistry 58(14): 8430-8436.
- Zhang J, Gao W, Liu Z, Zhang Z, Liu C, et al. (2014) Systematic analysis of main constituents in rat biological samples after oral administration of the methanol extract of fructus aurantii by HPLC-ESI-MS/MS. Iranian Journal of Pharmaceutical Research 13 (2): 493-503.
- Yin L, Cheng W, Qin Z, Yu H, Yu Z, et al. (2015) Effects of naringin on proliferation and osteogenic differentiation of human periodontal ligament stem cells in vitro and in vivo. Stem Cells International 2015: 758706.
- Zhao BT, Kim EJ, Son KH, Son JK, Min BS, et al. (2015) Quality evaluation and pattern recognition analyses of marker compounds from five medicinal drugs of Rutaceae family by HPLC/PDA. Archives of pharmacal research 38(8): 1512-1520.
- Chtourou Y, Gargouri B, Kebieche M, Fetoui H (2015) Naringin abrogates cisplatin-induced cognitive deficits and cholinergic dysfunction through the down-regulation of AChE expression and iNOS signaling pathways in hippocampus of aged rats. Journal of Molecular Neuroscience 56(2): 349-362.
- Renugadevi J, Prabu SM (2009) Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 256(1-2): 128-134.
- Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, et al. (2003) Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clinical Nutrition 22(6): 561-568.
- Choudhury R, Chowrimootoo G, Srai K, Debnam E, Rice Evans CA (1999) Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochemical and biophysical research communications 265(2): 410-415.
- Kim YH, Tabata Y (2015) Dual-controlled release system of drugs for bone regeneration. Advanced drug delivery reviews 94: 28-40.
- Cordenonsi LM, Bromberger NG, Raffin RP, Scherman EE (2016) Simultaneous separation and sensitive detection of naringin and naringenin in nanoparticles by chromatographic method indicating stability and photodegradation kinetics. Biomedical chromatography 30(2): 155-162.
- Chen KY, Lin KC, Chen YS, Yao CH (2013) A novel porous gelatin composite containing naringin for bone repair. Evidence-Based Complementary and Alternative Medicine 2013: 283941.
- Wang DM, Yang YJ, Zhang L, Zhang X, Guan FF, et al. (2013) Naringin enhances CaMKII activity and improves long-term memory in a mouse model of Alzheimer’s disease. International Journal of Molecular Sciences 14(3): 5576-5586.
- Cvetnic Z, Vladimir Knezevic S (2004) Antimicrobial activity of grapefruit seed and pulp ethanolic extract. Acta Pharm 54(3): 243-250.
- Choi MS, Do KM, Park YS, Jeon SM, Jeong TS, et al. (2001) Effect of naringin supplementation on cholesterol metabolism and antioxidant status in rats fed high cholesterol with different levels of vitamin E. Annals of Nutrition and Metabolism 45(5): 193-201.
- Suseem SR, Joseph D (2019) The myth and the fact on naringin-A review. Res J Pharm Tech 12: 367-374.
- Zhang H, Wong CW, Coville PF, Wanwimolruk S (2000) Effect of the grapefruit flavonoid naringin on pharmacokinetics of quinine in rats. Drug metabolism and drug interactions 17(1-4): 351-364.
- Sudto K, Pornpakakul S, Wanichwecharungruang S (2009) An efficient method for the large-scale isolation of naringin from pomelo (Citrus grandis) peel. International journal of food science & technology 44(9): 1737-1742.
- Zhao P, Duan L, Guo L, Dou LL, Dong X, et al. (2015) Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chemistry 173: 54-60.
- Pereira RM, Andrades NE, Paulino N, Sawaya AC, Eberlin MN, et al. (2007) Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules 12(7): 1352-1366.
- Guleria P, Kumar V (2017) Understanding the phenylpropanoid pathway for agronomical and nutritional improvement of mungbean. The Journal of Horticultural Science and Biotechnology 92(4): 335-348.
- Wang CH, Yu J, Cai YX, Zhu PP, Liu CY, et al. (2016) Correction: Characterization and Functional Analysis of 4-Coumarate: CoA Ligase Genes in Mulberry. PloS one 11(6): e0157414.
- Winkel Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant physiology 126(2): 485-493.
- Wong KC, Pang, WY, Wang XL, Mok SK, Lai WP, et al. (2013) Drynaria fortunei-derived total flavonoid fraction and isolated compounds exert oestrogen-like protective effects in bone. British journal of nutrition 110(3): 475-485.
- Wei M, Yang Z, Li P, Zhang Y, Sse WC, et al. (2007) Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. The American Journal of Chinese Medicine 35(04): 663-667.
- Li F, Sun X, Ma J, Ma X, Zhao B, et al. (2014) Naringin prevents ovariectomy-induced osteoporosis and promotes osteoclasts apoptosis through the mitochondria-mediated apoptosis pathway. Biochemical and Biophysical Research Communications 452(3): 629-635.
- Habauzit V, Sacco SM, Gil Izquierdo A, Trzeciakiewicz A, Morand C, et al. (2011) Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone 49(5): 1108-1116.
- Li N, Xu Z, Wooley PH, Zhang J, Yang SY, et al. (2014) Therapeutic potentials of naringin on polymethylmethacrylate induced osteoclastogenesis and osteolysis, in vitro and in vivo assessments. Drug design, development, and therapy 8: 1-11.
- Chen LL, Lei LH, Ding PH, Tang Q, Wu YM, et al. (2011) Osteogenic effect of Drynariae rhizoma extracts and Naringin on MC3T3-E1 cells and an induced rat alveolar bone resorption model. Archives of oral biology 56(12): 1655-1662.
- Jagetia GC, Reddy TK (2002) The grapefruit flavanone naringin protects against the radiation-induced genomic instability in the mice bone marrow: a micronucleus study. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 519(1-2): 37-48.
- Hu Y, Wei M, Niu Q, Ma R, Li Y, et al. (2019) Grape seed proanthocyanidin extract alleviates arsenic-induced lung damage through NF-κB Experimental Biology and Medicine 244(3): 213-226.
- Bacanlı M, Başaran AA, Başaran N (2015) The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food and chemical Toxicology 81: 160-170.
- Dhanya R, Arun KB, Nisha VM, Syama HP, Nisha P, et al. (2015) Preconditioning L6 muscle cells with naringin ameliorates oxidative stress and increases glucose uptake. PLoS One 10(7): e0132429.
- Chen F, Zhang N, Ma X, Huang T, Shao Y, et al. (2015) Naringin alleviates diabetic kidney disease through inhibiting oxidative stress and inflammatory reaction. PLoS One 10(11): e0143868.
- Golechha M, Sarangal V, Bhatia J, Chaudhry U, Saluja D, et al. (2014) Naringin ameliorates pentylenetetrazol-induced seizures and associated oxidative stress, inflammation, and cognitive impairment in rats: possible mechanisms of neuroprotection. Epilepsy & Behavior 41: 98-102.
- A Bakheet S, M Attia S (2011) Evaluation of chromosomal instability in diabetic rats treated with naringin. Oxidative medicine and cellular longevity 2011: 365292.
- Mahmoud AM, Ashour MB, Abdel Moneim A, Ahmed OM (2012) Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. Journal of Diabetes and its Complications 26(6): 483-490.
- Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, et al. (2014) Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. Journal of biomedical research 28(2): 132-145.
- Rehman K, Khan II, Akash MSH, Jabeen K, Haider K (2020) Naringenin downregulates inflammation‐mediated nitric oxide overproduction and potentiates endogenous antioxidant status during hyperglycemia. J Food Biochem 44(10): e13422.
- Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin S (2007) Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β Clinical & Experimental Immunology 147(2): 227-235.
- Manthey JA, Guthrie N, Grohmann K (2001) Biological properties of citrus flavonoids pertaining to cancer and inflammation. Current medicinal chemistry 8(2): 135-153.
- Benavente-Garcia O, Castillo J (2008) Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. Journal of agricultural and food chemistry 56(15): 6185-6205.
- Wang SS, Liu L, Zhu L, Yang YX (2011) Inhibition of TNF-alpha/IFNgamma induced RANTES expression in HaCaT cell by naringin. Pharmaceutical Biology 49(8): 810-814.
- Liu Y, Wu H, Nie YC, Chen JL, Su WW, et al. (2011) Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-κB pathway. International Immunopharmacology 11(10): 1606-1612.
- Luo YL, Zhang CC, Li PB, Nie YC, Wu H, et al. (2012) Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int immunopharmacol 13(3): 301-307.
- Gopinath K, Sudhandiran G (2012) Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 227: 134-143.
- Lewinska A, Siwak J, Rzeszutek I, Wnuk M (2015) Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol In Vitro29(3): 417-425.
- Attia SM (2008) Abatement by naringin of lomefloxacin-induced genomic instability in mice. Mutagenesis23(6): 515-521.
- Ikemura M, Sasaki Y, Giddings JC, Yamamoto J (2012) Preventive effects of hesperidin, glucosyl hesperidin and naringin on hypertension and cerebral thrombosis in stroke‐prone spontaneously hypertensive rats. Phytotherapy Research26(9): 1272-1277.
- Fallahi F, Roghani M, Moghadami S (2012) Citrus flavonoid naringenin improves aortic reactivity in streptozotocin-diabetic rats.Indian J Pharmacol 44(3): 382-386.
- Jeon SM, Bok SH, Jang MK, Lee MK, Nam KT, et al. (2001) Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life sciences69(24): 2855-2866.
- Duarte N, Lage H, Abrantes M, Ferreira MJU (2010) Phenolic compounds as selective antineoplasic agents against multidrug-resistant human cancer cells. Planta medica 76(10): 975-980.
- Jäger AK, Saaby L (2011) Flavonoids and the CNS. Molecules 16(2): 1471-1485.
- Sachdeva AK, Kuhad A, Chopra K (2014) Naringin ameliorates memory deficits in experimental paradigm of Alzheimer's disease by attenuating mitochondrial dysfunction. Pharmacology Biochemistry and Behavior127: 101-110.
- Jung UJ, Kim SR (2014) Effects of naringin, a flavanone glycoside in grapefruits and citrus fruits, on the nigrostriatal dopaminergic projection in the adult brain. Neural Regeneration Research9(16): 1514.
- Jeong KH, Jung UJ, Kim SR (2015) Naringin attenuates autophagic stress and neuroinflammation in kainic acid-treated hippocampus in vivo. Evid Based Complement Alternat Med 2015: 354326.
- Wang D, Yan J, Chen J, Wu W, Zhu X, et al. (2015) Naringin improves neuronal insulin signaling, brain mitochondrial function, and cognitive function in high-fat diet-induced obese mice. Cell Mol Neurobiol 35(7): 1061-1071.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.